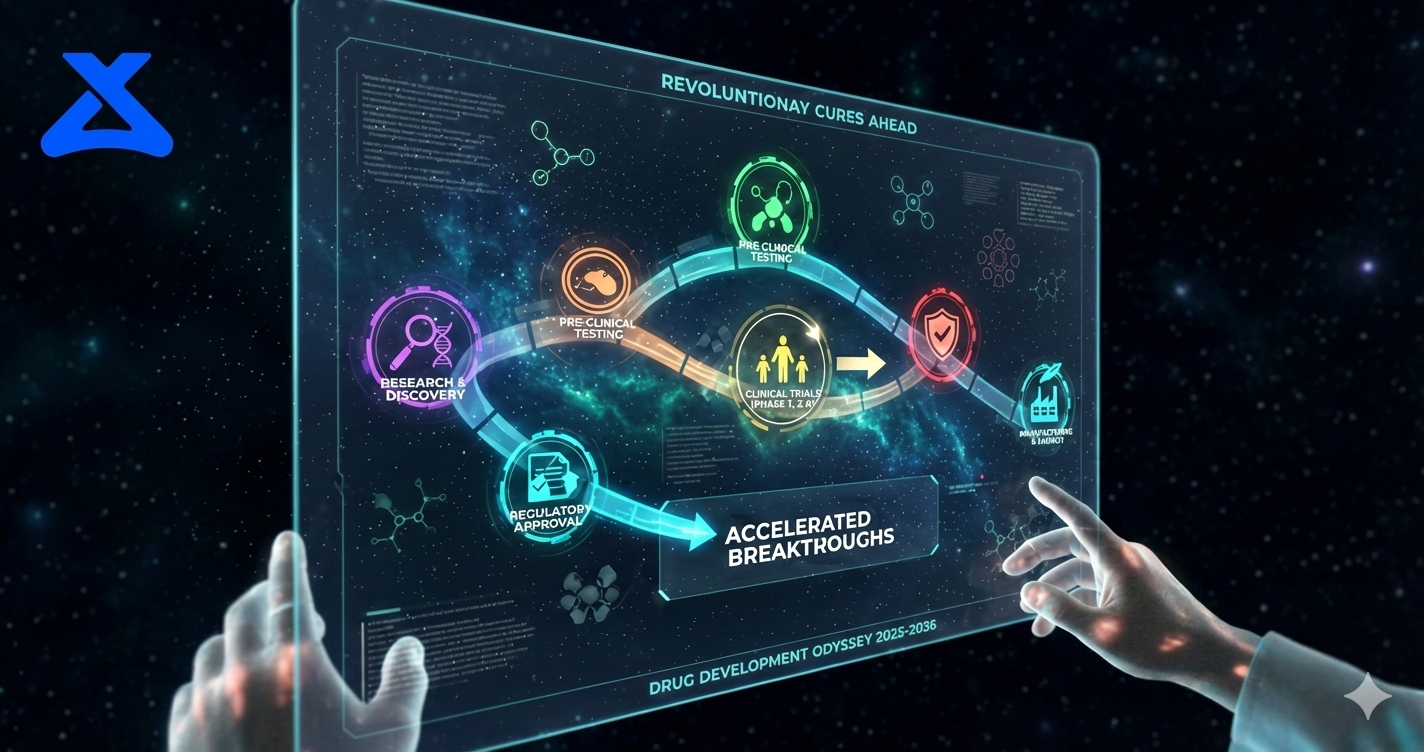
Accelerating science from
Lab to Market
Strategic solutions for every stage of your preclinical development.
Pharmacology & Toxicology
Safety proved. Efficacy defined. We transform complex pharmacology and toxicology data into a clear regulatory roadmap to de-risk your lead candidates and move toward the clinic with confidence.
Global Regulatory Affairs
Navigate complex regulatory pathways with expert guidance for FDA, EMA, and international submissions.
EU Public Affairs: European-funded Health Projects
Strategic support for accessing European funding and managing collaborative research initiatives.
Sourcing and Management
End-to-end project coordination from preclinical design to regulatory approval and market entry.
Driving your scientific advancement
Agile preclinical development from the lab bench to the global market
Strategic solutions for every stage of your preclinical development.
What drives our acceleration
Strategic Acceleration
We reduce the time between discovery and real-world application, optimizing every preclinical/non-clinical phase with methods that drive the transition from laboratory to market.
Regulatory Rigor
We guide biotechnology companies, SMEs, and research centers through complex regulatory environments, transforming each requirement into a strategic opportunity that strengthens development strengthens regulatory milestones.
Science–Industry Bridge
We unite research, clinical strategy, and market vision, acting as the bridge that converts scientific potential into safe, differentiated, high-impact solutions.
Vision and Precision
We provide clarity and confidence at every step: expert analysis, precise planning, and a global perspective that ensure solid decisions and results with excellence.
Ready to accelerate your development?
Discover how our strategic consulting can transform your preclinical program into accelerated market approvals
Why Choose Axeleros
Our commitment to excellence sets us apart in the preclinical development landscape
International network spanning Europe, Americas, and Asia-Pacific with deep understanding of regulatory requirements across regions.
Rigorous scientific methodology combined with strategic thinking to ensure robust, defensible data that meets regulatory standards.
Up-to-date knowledge of evolving regulatory landscapes, ensuring your program stays ahead of compliance requirements.
Optimized processes and parallel development strategies that reduce timelines without compromising quality or compliance.
GLP-compliant operations and robust quality systems that ensure data integrity and regulatory acceptance.
Collaborative approach where your success is our success, providing transparent communication and strategic guidance throughout.
Find out more content in our Blog
Ver todas publicaciones »Drop us a message today!
Our team of preclinical development experts is ready to discuss your project needs and explore how we can support your journey from laboratory to market.
We are here to help!
info@axelerospreclinical.com
Brussels, Belgium